Endothermic reactions only.
Neither exothermic or endothermic reactions.
Both exothermic and endothermic reactions.
Exothermic reactions only.
Increased temperature and decreased concentration of reactants
Increased temperature and increased concentration of reactants
Decreased temperature and decreased concentration of reactants
Decreased temperature and increased concentration of reactants.
Number of molecular collisions will increase
Temperature will increase
Forward reaction is endothermic
Reaction will shift to the left
By increasing the kinetic energy of the molocules
By reducing the activiation energy
By increasing the activation energy
By providing a large surface area
Temperature
Molocule size
X ray absorption
Absorptivity
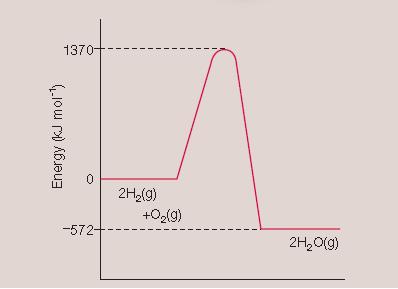
572 kJ mol-1
1370 kJ mol-1
-572 kJ mol -1
1942 kJ mol -1
Endothermic reactions only.
Neither exothermic or endothermic reactions.
Both exothermic and endothermic reactions.
Exothermic reactions only.
Both salts are endothermic.
Both salts are exothermic.
NH4NO3 is exothermic and the dissolving of NaOH is endothermic.
NH4NO3 is endothermic and the dissolving of NaOH is exothermic.
Number of molecular collisions will increase
Temperature will increase
Forward reaction is endothermic
Reaction will shift to the left
True
False
True
False
Yes
No
A
B
C
D
E
Catalyst, Product Concentration, Container Volume
Temperature, Reactant Concentration, Pressure
Temperature, Reactant Concentration, Catalyst
Temperature, Product Concentration, Container Volume
25 M/min
10 M/min
2.5 M/min
5.0 M/min
The rate of formation of ammonia is two thirds the rate of consumption of hydrogen
The rate of formation of ammonia is half the rate of consumption of hydrogen
The rate of formation of ammonia is equal to the rate of consumption of hydrogen
The rate of formation of ammonia is 1/5 times the rate of consumption of hydrogen
3
4
2
1
Heat of fusion
Heat of reaction
Free energy
Activation energy
30.8 sec
0.0800 sec
0.0554 sec
61.5
Ln[A] versus t if first-order in A
Ln[A] versus t if second-order in A
1/[A] versus t if first-order in A
125 kJ/mol
-75 kJ/mol
75 kJ/mol
-125 kJ/mol
Adding a suitable catalyst
Increasing the concentrations of reactants
Raising the temperature of the reaction
All of these options will lower the activation energy for a reaction
There is no way to lower the activation energy of a reaction
Rate=k[NO2]
Rate=k[NO2][CO]
Rate=k[NO2]^2[CO]
Rate=k[NO2]^2
NLSC. Chemistry-Engaging Assignments for the New Lower Secondary Curriculum Assignment 1: Chemical Bonding Scenario: You…
4(a) what are your roles as citizen of Uganda? (b) Each and every individual in…
3(a) why do we political Eduction in the New Uganda curriculum? (b) Explain the roles…
Leave a Comment