( i ) Atomic number .
( ii ) Mass number .
b ) The electronic structure of some selected elements are :
| Element | Electronic structure |
| A | 2,8,2 |
| B | 2,8,6 |
| C | 2,1 |
| D | 2,8 |
| E | 2,6 |
( i ) Which element belongs to group II of the Periodic Table ? Give a reason for your answer .
( ii ) Which elements belong to the same group in the Periodic Table ?
(iii ) Which of the elements has the highest proton number ?
(vi) State how you arrived at the answer .
( iv ) Draw the electron structure of element E
( v ) Write the formula of the most likely ion formed by element B
( vi ) State two physical properties of element A that differentiates it from element E.
2. Figure 3.7 is an outline of part of the Periodic Table with some elements shown .
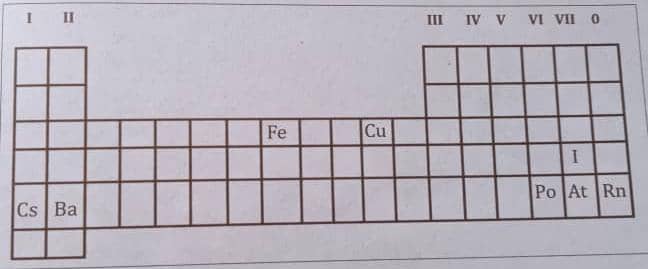
From your knowledge of periodic trends in the properties of elements , answer the following questions :
a ) Which element would have an electronic structure in which the outermost shell :
( i ) has 2 electrons ? ( ii ) is fully filled ?
b ) Cu is used for making coins while Cs or Ba cannot be used for this purpose . Why not ?
c ) Element X ( not indicated on the table above ) has the electronic structure 2 , 8 , 6 .
( i) Locate the position of X on the Periodic Table ( its group and period ) .
( ii ) Is X a metal or non – metal ?
(iii) Name one element (not shown on the table above) which you expect to have chemical properties similar to those of X.
NLSC. Chemistry-Engaging Assignments for the New Lower Secondary Curriculum Assignment 1: Chemical Bonding Scenario: You…
4(a) what are your roles as citizen of Uganda? (b) Each and every individual in…
3(a) why do we political Eduction in the New Uganda curriculum? (b) Explain the roles…
Leave a Comment