To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions.
The technical storage or access is strictly necessary for the legitimate purpose of enabling the use of a specific service explicitly requested by the subscriber or user, or for the sole purpose of carrying out the transmission of a communication over an electronic communications network.
The technical storage or access is necessary for the legitimate purpose of storing preferences that are not requested by the subscriber or user.
The technical storage or access that is used exclusively for statistical purposes.
The technical storage or access that is used exclusively for anonymous statistical purposes. Without a subpoena, voluntary compliance on the part of your Internet Service Provider, or additional records from a third party, information stored or retrieved for this purpose alone cannot usually be used to identify you.
The technical storage or access is required to create user profiles to send advertising, or to track the user on a website or across several websites for similar marketing purposes.
SOIL
Soil is the loose surface material consisting of inorganic particles and organic matter that covers most of the land surface. Soil provides the structural support and the source of water and nutrients for plants used in agriculture.
Introduction
Soils vary greatly in their chemical and physical properties which depend on their age and on the conditions (parent material, climate, topography and vegetation) under which they were formed.
Processes such as leaching, weathering and microbial activity combine to make a whole range of different soil types, each of which has particular strengths and weaknesses for agricultural production.
This Agriculture Note provides a brief introduction to soils and the major soil components.
Inorganic component
Inorganic material is the major component of most soils. It consists largely of mineral particles with specific physical and chemical properties which vary depending on the parent material and conditions under which the soil was formed. It is the inorganic fraction of soils which determines soil physical properties such as texture and has a large effect on structure, density and water retention.
Soil texture
The texture of soil is a property which is determined largely by the relative proportions of inorganic particles of different sizes.
The following five size fractions are used to describe the inorganic fraction of soils:
Sand
Quartz is the predominant mineral in the sand fraction of most soils. Sand particles have a relatively small surface area per unit weight, low water retention and little chemical activity compared with silt and clay.
Silt
Silt has a relatively limited surface area and little chemical activity. Soils high in silt may compact under heavy traffic and this affects the movement of air and water in the soil.
Clay
Clays have very large surface areas compared with the other inorganic fractions. As a result clays are chemically very active and are able to hold nutrients on their surfaces. These nutrients can be released into soil water from where they can be used by plants. Like nutrients, water also attaches to the surfaces of clays but this water can be hard for plants to use.
There are many different types of clays. The ability of clays to swell and to retain a shape into which they have been formed, as well as their sticky nature, distinguish them from sand and silt.
Soil textural class
The relative proportion of sand, silt and clay particles determines the physical properties of soil including the texture. The surface area of a given amount of soil increases significantly as the particle size decreases. Consequently, the soil textural class also gives an indication of some soil chemical properties.
The exact proportions of sand, silt and clay in a soil can only be determined in a laboratory but a naming system has been developed to approximately describe the relative proportions of sand, silt and clay in soil. This classification of soil can be undertaken in the field where particular properties indicate possible textural classes.
To estimate texture in the field, crush a small sample of soil (10 to 20g) in one hand. After removing any gravel or root matter, work the soil in the fingers to break down any aggregates which may be present. With the sample moist but not sticky, the textural class can be estimated by the feel of the sample between the fingers
Textural class description
Sand – A sand has a loose gritty feel and does not stick together. Individual sand grains can be seen or felt.
Loamy Sand – In a loamy sand particles barely stick together and a moulded piece of soil just holds its shape.
Sandy Loam – A sandy loam sticks together more than a loamy sand but can be easily broken. Individual sand grains can be felt and heard if a wet sample is rubbed between the index finger and thumb and held close to the ear.
Silty Loam – A silty loam is like a loam but has a smooth silky feel when a moist sample is pushed between the index finger and thumb. On drying a sample can form a hard lump but this may be broken by hand.
Loam – A loam breaks into crumbs but will tend to stick together. Sand grains cannot be felt in a moist sample which when squeezed will retain its shape when handled freely. Loams are usually soft to the feel.
Sandy Clay Loam – A sandy clay loam is like a clay loam but sand grains can be felt (and heard – see Sandy Loam)
Silty Clay Loam – A silty clay loam is like a clay loam but silty as well and smooth to the touch.
Clay Loam – More easily moulded into a shape than a loam, a clay loam rolls out to a thin ribbon between the palms while a loam will break-up. When dry a clay loam will form a lump but is not as tough to break as a clay.
Sandy Clay – A sandy clay is like a clay but sand grains can be felt (and heard – see Sandy Loam).
Silty Clay – A silty clay is like a clay but smoother.
Clay – Clays are tough and can be moulded into shapes when moist. Clays form a long flexible ribbon when rubbed between the palms and the ribbon can often be bent into a “U” shape without breaking. Clays dry into very hard clods.
It should always be remembered that soil texture often varies with depth and that the properties of the topsoil are affected by the properties of the subsoil.
Structure.
Structure is the arrangement of soil particles and the pore spaces between them. A soil with structure which is beneficial to plant growth has stable aggregates between 0.5 and 2 mm in diameter. Such soils have good aeration and drainage.
Chemical properties
The inorganic minerals of soils consist primarily of silicon, iron and aluminium which do not contribute greatly to the nutritional needs of plants. Those in the clay fraction have the capacity to retain nutrients in forms which are potentially available for plants to use.
Organic component
The organic matter of soil usually makes up less than 10% by weight of soil. It can be subdivided into the living and the non-living fractions. The non-living fraction contributes to the soil’s ability to retain water and some nutrients and to the formation of stable aggregates.
Organic matter
The organic matter fraction of soils comes from the decomposition of animal or plant products such as faeces and leaves. Soil organic matter contributes to stable soil aggregates by binding soil particles together.
Plants living in soil continually add organic matter in the form of roots and debris. Decomposition of this organic matter by microbial activity releases nutrients for the growth of other plants.
The organic matter content of a soil depends on the rates of organic matter addition and decomposition. Soil micro-organisms are the primary agents responsible for the decomposition of organic matter such as plant residues. Initially, the sugars, starch and certain proteins are readily attacked by a number of different micro-organisms. The more resistant structural components of the cell wall are decomposed relatively slowly. The less easily decomposed compounds, such as lignin and tannin, impart a dark colour to soils containing a significant organic matter content.
The decomposition rate of organic materials depends on how favourable the soil environment is for microbial activity. Higher decomposition rates occur where there are warm, moist conditions, good aeration, a favourable ratio of nutrients, a pH near neutral and freedom from toxic compounds.
Soil organisms
The soil contains numerous organisms ranging from microscopic bacteria to large soil animals such as earthworms. The soil micro-organisms include bacteria, fungi, actinomycetes, algae, protozoa and nematodes.
The diversity of soil organisms can both assist and hinder plant growth. Beneficial activities include organic matter decomposition, nitrogen fixation, transformation of essential elements from one form to another, improvement in soil structure through soil aggregation, and improved drainage and aeration.
Under some circumstances soil organisms compete with plants for nutrients.
Bacteria are the smallest and most numerous micro-organisms in the soil.
They make an important contribution to organic matter decomposition, nitrogen fixation and the transformation of nitrogen and sulphur.
The fungi and actinomycetes contribute beneficially to organic matter decomposition. The group of large soil animals includes earthworms, which incorporate organic matter into the soil as well as improving aeration and drainage by means of their channels. Some soil fungi, nematodes, and insects feed on roots and lateral shoots to the detriment of plants.
Soil acidity.
Soil acidity is a natural and induced chemical condition of soils that can:
The process of soil acidification is a potentially serious land degradation issue. Without treatment, soil acidification will have a major impact on agricultural productivity and sustainable farming systems and acidification can also extend into subsoil layers posing serious problems for plant root development and remedial action.
In some regions, there has been a drop of one pH unit over the last 20 to 30 years. Already, some farming areas have lost the ability to grow preferred agricultural species such as phalaris and lucerne simply because, without lime, the soil is too acid.
Understanding soil acidity
Soil acidity is a natural and induced chemical condition of soils that can:
The process of soil acidification is a potentially serious land degradation issue. Without treatment, soil acidification will have a major impact on agricultural productivity and sustainable farming systems and acidification can also extend into subsoil layers posing serious problems for plant root development and remedial action.
In some regions, there has been a drop of one pH unit over the last 20 to 30 years. Already, some farming areas have lost the ability to grow preferred agricultural species such as phalaris and lucerne simply because, without lime, the soil is too acid.
Soil acidity occurs naturally in higher rainfall areas and can vary according to the landscape geology, clay mineralogy, soil texture and buffering capacity. Soil acidification is a natural process, accelerated by some agricultural practices .
When plant material is removed from the paddock, alkalinity is also removed. This increases soil acidity. When grain, pasture and animal products are harvested from a paddock, the soil is left more acid. Hay removal is particularly acidifying because large amounts of product are removed.
More significantly, soil acidification is most often a result of nitrate leaching. Nitrogen is added to the soil in a number of ways:
Acidification occurs in agricultural soils as a result of the:
Soil pH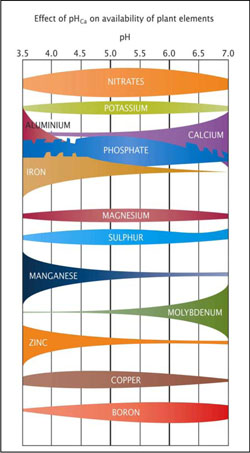
Soil pH is a measure of acidity or alkalinity. A pH of 7 is neutral, above 7 is alkaline and below 7 is acid. Because pH is measured on a logarithimc scale, a pH of 6 is 10 times more acid than a pH of 7. Soil pH can be measured either in water (pHw) por in calcium chloride (pHCa) and the pH will vary depending on the method used. As a general rule, pH measured in calcium chloride is 0.7 of a pH unit lower than pH measured in water. When a laboratory measures your soil’s pH it is important that they specify which method (water or calcium chloride) was used.
For most acid soils, the most practical management option is to add lime to maintain current soil pH status or increase surface soil pH.
The acid attack.
Acidity itself is not responsible for restricting plant growth. The associated chemical changes in the soil can restrict the availability of essential plant nutrients (for example, phosphorus, molybdenum) and increase the availability of toxic elements (for example, aluminium, manganese). Essential plant nutrients can also be leached below the rooting zone. Biological processes favourable to plant growth may be affected adversely by acidity.
Bacterial populations generally prefer a slightly acid environment. However highly acidic soils can inhibit the survival of useful bacteria, for example the rhizobia bacteria that fix nitrogen for legumes. As the soil acidifies, the favorable environment for bacteria, earthworms and many other soil organisms is degraded. Acid soils have a major effect on plant productivity once the soil pHCa falls below 5:
Soil pH will influence both the availability of soil nutrients to plants and how the nutrients react with each other. At a low pH many elements become less available to plants, while others such as iron, aluminum and manganese become toxic to plants and in addition, aluminum, iron and phosphorus combine to form insoluble compounds. In contrast, at high pH levels calcium ties up phosphorus, making it unavailable to plants, and molybdenum becomes toxic in some soils. Boron may also be toxic at high pH levels in some soils.
The relative availability of 12 essential plant nutrients in well-drained mineral soils in temperate regions in relation to soil pH. A pHCa range between 5 and 6 (between heavy lines) is considered ideal for most plants.
Soil testing
Soil test may refer to one or more of a wide variety of soil analysis conducted for one of several possible reasons. Possibly the most widely conducted soil tests are those done to estimate the plant-available concentrations of plant nutrients, in order to determine fertilizer recommendations in agriculture. Other soil tests may be done for engineering (geotechnical), geochemical or ecological investigations.
Soil testing is often performed by commercial labs that offer a variety of tests, targeting groups of compounds and minerals. The advantages associated with local lab is that they are familiar with the chemistry of the soil in the area where the sample was taken. This enables technicians to recommend the tests that are most likely to reveal useful information.
The amount of plant available soil phosphorus is most often measured with a chemical extraction method, and different countries have different standard methods. Just in Europe, more than 10 different soil P tests are currently in use and the results from these tests are not directly comparable with each other.
Do-it-yourself kits usually only test for the three “major nutrients”, and for soil acidity or pH level. Do-it-yourself kits are often sold at farming cooperatives, university labs, private labs, and some hardware and gardening stores. Electrical meters that measure pH, water content, and sometimes nutrient content of the soil are also available at many hardware stores. Laboratory tests are more accurate than tests with do-it-yourself kits and electrical meters. Here is an example soil sample report from one laboratory.
Soil testing is used to facilitate fertilizer composition and dosage selection for land employed in both agricultural and horticultural industries.
Prepaid mail-in kits for soil and ground water testing are available to facilitate the packaging and delivery of samples to a laboratory. Similarly, in 2004, laboratories began providing fertilizer recommendations along with the soil composition report.
Lab tests are more accurate and often utilize very precise flow injection technology, though both types are useful. In addition, lab tests frequently include professional interpretation of results and recommendations. Always refer to all proviso statements included in a lab report as they may outline any anomalies, exceptions, and shortcomings in the sampling and/or analytical process/results.
Some laboratories analyze for all 13 mineral nutrients and a dozen non-essential, potentially toxic minerals utilizing the “universal soil extractant” (ammonium bicarbonate DTPA).
Understanding soil pH by testing
Soil pH is one of the most routinely measured soil parameters. It is used as a benchmark to interpret soil chemical processes and governs the availability of many essential or toxic elements for plant growth.
Soil pH is a common measure of the soil’s acidity or alkalinity because:
Field test kits are available that use colour to indicate pH levels. The kits are inexpensive, easy to use and will test a lot of samples but should not be relied on for decisions such as rates of lime application. Test kits will only tell you whether your soil is acid or alkaline.
A number of compact testing meters that can be used out in the paddock are available, most of which are capable of giving accurate results if used correctly. Professional soil analysis is recommended and sending soil samples to a recognised laboratory ensures the most accurate results.
Testing of both topsoil and subsoil is recommended. When interpreting plant responses based on soil pH, the surface (A horizon) and sub-surface (B horizon) need to be considered.
The soil pHW is considered to be closer to the pH that the plant roots experience in the soil. But it is subject to large variation within the paddock because of seasonal changes in soil moisture and the ionic concentration of the soil solution that is related to the amount of total salts in the soil.
Research has shown that seasonal variation of pHW can vary up to 0.6 of a pH unit in any one year. In comparison, the measurements of soil pHCa is less affected by seasons.
Farmers can take soil samples at different times during the year without affecting the final diagnosis or interpretation.
Soil pHCa measurements vary from pHCa 3.6 to pHCa 8 for a range of different soil textures (sandy loams to heavy clays). Soil pHW values lie between pHW 4 and pHW 9.
Higher pHW values to around 10 may be associated with alkali mineral soils containing sodium carbonates and bicarbonates.
Compost
Compost is the result of the natural rotting process that occurs with all organic material. The increasing suburbanisation in countries plus the expansion of the nursery industry has produced a huge demand for compost. Compost is a valuable source of organic matter for soils and contains nutrients that are slowly available for plant growth.
Composting reduces the amount of wastes going to landfill and recovers valuable organic matter for our soils. Governments and business have seen these opportunities, and a whole new industry has been born – commercial composting. Commercial producers are simply speeding up the natural process of rotting. By creating the right environment, the rotting process of any organic material can, not only be accelerated, but also manipulated, to produce the desired results.
The development of new markets for compost is a strong focus of the industry at present. Like any new business, it is critical that marketing studies are completed well before any capital is spent on establishing a composting enterprise.
Physical requirements
The basic ingredients of making compost are quite simple: – a supply of organic materials, microorganisms, moisture and oxygen. The last three are universally available and practically free, however, watering costs must be considered in every climate. Compost is a net user of water. Organic material can be sourced from anywhere – garden or green waste, or animal manure are the usual sources but you can compost kitchen waste, sawdust and even paper. Successful producers are composting green wastes, grease trap sludges, food wastes, sawdust, manures and wool scour wastes.
Depending on your method of production, you may also need to add some other materials such as gypsum, lime, dolomite and even soil. The other requirement is machinery.
Depending on the scale of production and the types of waste accepted, composting requires the use of heavy machinery. As a minimum, commercial scale operations require the use of a front-end loader to load, unload and turn compost. Screening equipment for the end product is also typical for most operations. Finally, you need microorganisms to do the composting. These occur naturally so all you need to do is provide the right environment to encourage the right types of microbes.
Farm water
Farm water, also known as agricultural water, is water committed for use in the production of food and fiber. On average, 80 percent of the fresh water withdrawn from rivers and groundwater is used to produce food and other agricultural products. Farm water may include water used in the irrigation of crops or the watering of livestock.
Livestock water use
Livestock and meat production have one of the largest water footprints of the agricultural industry. Taking nearly 6,814 litres of water to produce half a kilogram of beef and 2,180 litres for pork. For reference, it only takes about 409 litres gallons of water to harvest half a kilogram of maize grain.
Livestock production is also one of the most resource intensive agricultural outputs. This is largely due to their large feed conversion ratio. Livestock’s large water consumption may also be attributed to the amount of time it takes to raise an animal to slaughter. Again, in contrast to maize, which grows to maturity in about 100 days. This adds an extra 995 days of water to grow cattle. The global “food animal” population is just over 20 billion creatures, with 7+ billion humans, this equates to about 2.85 animals per human.
Poultry and fowl
Water is one of the most crucial aspects of poultry raising, as they use this to carry food through their system, and assist in digestion. Additionally, farmers monitor flock water consumption to measure the overall health of their birds. As birds grow older they should be consuming more feed and about three times as much water. In just three weeks a bird’s water consumption should increase by about 38 litres a day. Water consumption is also influenced by temperature. When it is hot outside bird will pant to keep cool, thus losing much of their water.
Horticulture water use
Irrigation
Irrigation is the artificial application of water to land for the purpose of agricultural production. Effective irrigation will influence the entire growth process from seedbed preparation, germination, root growth, nutrient utilisation, plant growth and regrowth, yield and quality.
The key to maximising irrigation efforts is uniformity. The producer has a lot of control over how much water to supply and when to apply it but the irrigation system determines uniformity. Deciding which irrigation systems is best for your operation requires a knowledge of equipment, system design, plant species, growth stage, root structure, soil composition, and land formation. Irrigation systems should encourage plant growth while minimising salt imbalances, leaf burns, soil erosion, and water loss. Losses of water will occur due to evaporation, wind drift, run-off and water (and nutrients) sinking deep below the root zone.
Proper irrigation management takes careful consideration and vigilant observation.
The value of irrigation
Irrigation allows primary producers,
Types of Irrigation Systems
Irrigation system description
Choosing an irrigation system
There is a huge diversity in the types of irrigation technologies/systems used, which is attributable to,
Source of irrigation water
The vast majority of irrigation water use is pumped directly from a water source (river, creek, channel, drag line, hole, dam or bore).
Irrigation scheduling
Irrigation scheduling is the process by which an irrigator determines the timing and quantity of water to be applied to the crop/pasture. The challenge is to estimate crop water requirements for different growth stages and climatic conditions.
To avoid over or under watering, it is important to know how much water is available to the plant, and how efficiently the plant can use it. The methods available to measure this include: (i) plant observation, (ii) feel and appearance of the soil, (iii) using soil moisture monitoring devices; or (iv) estimating available water from weather data.
At 75 % field capacity, (i) Sands and sandy loams – are slightly coherent, will form a weak ball under pressure but breaks easily, (ii) Loams, clay loams and clays- are coherent, soil has a slick feeling and ribbons easily, and will not roll into long thin rods 2.5 diameter, and (iii) Comment – there is adequate water and air for good plant growth
At 0-25 % field capacity (or wilting point), (i) Sands and sandy loams – are dry, loose, flows through fingers, (ii) Loams, clay loams and clays are crumbly and powdery, small lumps break into powder, and will not ball under pressure, and (iii) Comment – plants desperately need watering and will die soon.
Problems
While irrigation has provided a number of important benefits the potential drawbacks of over/under watering include,
Under-watering
Over-watering
You can ask our super AI Agriculture Teacher below any question of s5 and s6 agriculture and get answers